In this article, we will review heterogeneous catalysis and its applications in the chemical industry. Catalysis is the method in which a catalyst speeds up the pace of a chemical process. The majority of catalytic reactions are heterogeneous. Heterogeneous catalysts have some benefits over homogeneous catalysts. It is due to their ease of production, handling, separation from the reaction mixture, recovery, and reuse, as well as their stability, low cost, and low toxicity. This review will assist you in gaining a good knowledge of heterogeneous catalytic reactions.
Rosemary Salin, Cademix Institute of Technology
Catalysis and Its Significance
Catalysts play a crucial role in the body functions and processes that keep us alive. Without catalysts, which highly accelerate the pace of reaction in living organisms, life would not be sustained. These catalysts are also used in chemistry for manufacturing chemicals. A catalyst is a material that influences the rate or direction of a chemical process. It increases product yield, reduces material costs, and speeds up lengthy processes in chemical industries. However, the consumption of a catalyst in a reaction occurs very rarely. In fact, a catalyst speeds up the reaction by offering different routes to the products. The activation energy of each catalytic process is lower than that of the non-catalytic process. It can also affect the direction or selectivity of a reaction.
Catalysts play an important role in the synthesis of more than 85% of all chemical products. Furthermore, catalysis is directly responsible for 15–20% of economic activity in developed countries. This means that catalysis is economically significant. Moreover, catalysis is a green technology. That is, catalysis will aid in the reduction of byproducts. Besides, using catalysts on a large scale might save the planet a large amount of energy. As a result, it is also a method of conserving energy. The following section will help you to understand the importance of catalysis with a special emphasis on heterogeneous catalysis.
Homogeneous vs. Heterogeneous Catalysis
There are primarily two forms of catalysis: homogeneous and heterogeneous catalysis.
Homogeneous Catalysis
Homogeneous catalysis refers to reactions in which the catalyst and reactants are in the same phase. These catalysts are often found in the liquid phase. Acid and base-catalyzed aqueous solution reactions are homogeneous catalytic reactions. For example, the Oxo process for the synthesis of aldehydes from alkene is a homogeneous catalytic reaction. It is a method for the synthesis of aldehydes from alkenes. In this process, the catalyst is a liquid-phase cobalt or rhodium complex.
Heterogeneous Catalysis
Heterogeneous catalysis is catalysis in which the phase of the catalyst is different from that of the reactants or products. It usually involves solid-phase catalysts and gas-phase reactants. Surface catalysis is another name for this catalysis. The major advantage of using a heterogeneous catalyst is the ease with which the catalyst can be separated from the product stream. Furthermore, we can reuse the catalyst several times. As a result, heterogeneous catalytic chemical reactions more continuous and economically desirable. In addition, heterogeneous catalysts are often more resistant to harsh working conditions than their homogeneous counterparts. These benefits make heterogeneous catalysis preferable to homogeneous catalysis. As a result, heterogeneous catalysts play a major role in the synthesis of industrial chemicals. In the following sections, we will focus on heterogeneous catalysis. Additionally, we will review some major industrial applications of it.
Comparison of Heterogeneous and Homogeneous Catalysis| Homogeneous Catalysis | Heterogeneous Catalysis |
|---|---|
| Same phase as the reaction medium | Different phase as the reaction medium |
| Catalyst separation is difficult | Easy separation of the catalyst |
| Expensive and difficult to recycle | Readily regenerated and recycled |
| High selectivity | Low selectivity |
| Low reaction temperature | High reaction temperature |
| Easy modification of the catalyst | Relatively difficult modification of the catalyst |
| Short life and requires extensive purification step | Long life and less purification step |
Mechanistic Aspects of Heterogeneous Catalysis
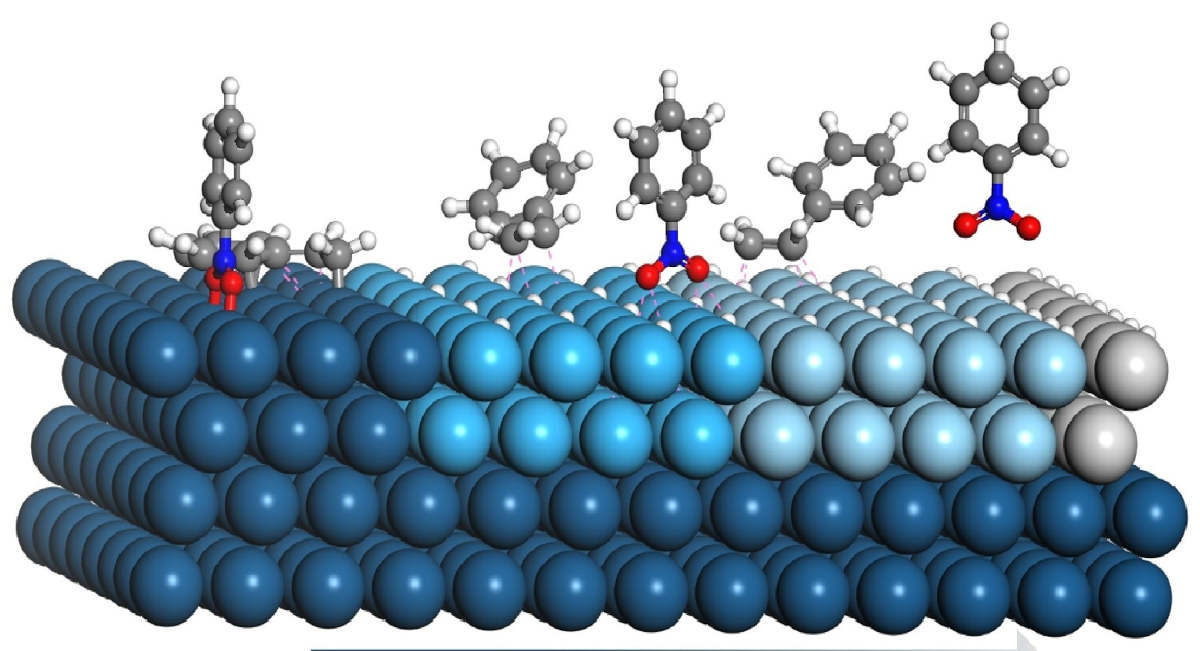
In heterogeneous catalysis, the reactants are typically gases and the catalyst is often solid. Due to the fact that the reaction begins at the surface of the solid catalyst, this is also referred to as surface catalysis. These catalysts have a very large surface area for interaction. You should have a fundamental understanding of the mechanisms of action of the catalysts before moving on to their industrial applications. Firstly, the reactants must first diffuse from the fluid phase to the solid surface. It is followed by three consecutive steps: adsorption, surface reaction, and desorption. Figure 2 is the schematic representation of the hydrogenation of ethene on a solid catalyst. It depicts the general mechanism of heterogeneous catalytic reactions.
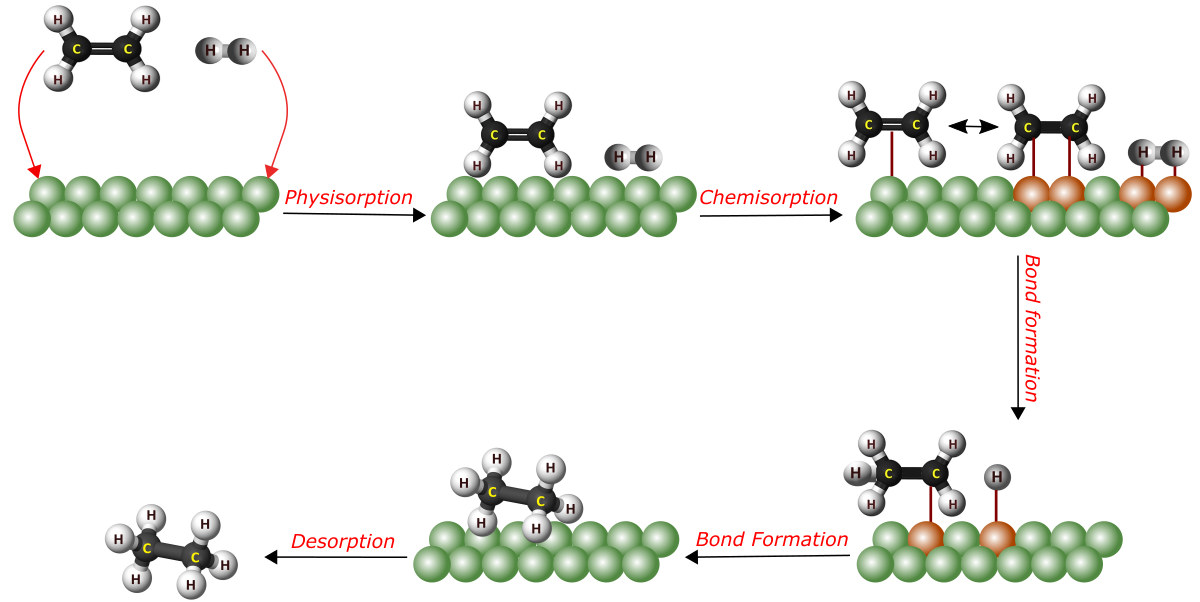
hydrogenation of ethene on a solid catalyst.
Adsorption
Adsorption is a necessary step in heterogeneous catalysis. It is the process by which a molecule in the gas or solution phase binds to a solid surface. There are two forms of adsorption: physisorption and chemisorption.
- Physisorption:
As the name indicates, it is physical adsorption. In physisorption, the molecule adheres to the surface through the action of van der Waals forces. Generally, physisorption occurs before chemisorption in heterogeneous catalysis as an intermediate energy state. - Chemisorption:
Chemisorption is a type of adsorption in which the surface and the adsorbate undergo a chemical interaction. As a result, new chemical bonds are formed at the adsorbent surface.
Surface Reactions
Adsorption sites are not usually active catalyst sites. Therefore, the reactant molecules must move over the surface to reach an active site. Then the reactant molecules will react at the active site to create product molecules. In fact, this product formation takes a more energy-efficient route via catalytic intermediates.
Desorption
Desorption is the final step in heterogeneous catalysis. It is the reverse of adsorption. That is, the product molecules will separate from the catalyst surface during desorption. Finally, the products will diffuse out from the solid surface into the fluid phase.
Heterogeneous Catalysis in Chemical Industry
As we have seen, heterogeneous catalytic reactions provide a number of benefits over homogeneous catalytic reactions. As a result, heterogeneous catalysis is a key component of many significant industrial processes. The following fields make extensive use of heterogeneous catalysts:
- synthesis of organic and inorganic chemicals;
- energy production;
- environmental protection.
We shall now discuss the use of heterogeneous catalysts in each of these domains separately.
Organic and Inorganic Chemical Synthesis

The synthesis of organic and inorganic compounds makes extensive use of heterogeneous catalysts. It is crucial because it makes it possible to manufacture products selectively and at a faster, larger scale. One of the most notable examples is the Haber-Bosch process for ammonia synthesis. This method uses a metal catalyst to convert atmospheric nitrogen (N2) to ammonia (NH3) through a reaction with hydrogen (H2) at high temperatures and pressures. In fact, the heterogeneous catalyst allows for the hydrogenation of N2, which is very reactive. Similarly, the hydrogenation of carbon monoxide tho methanol is of major industrial importance. Other examples of large-scale chemical synthesis using heterogeneous catalysts are the Contact process for sulfuric acid synthesis, Ziegler-Natta polymerization, the Ostwald process for nitric acid synthesis, and so on. Generally, metal catalysts are used in these reactions.
Energy Production
Metal catalysis is commonly employed in energy production processes. It is critical in the use of fossil fuels like petroleum, coal, and natural gas. For instance, in the processing of crude oil, heterogeneous catalyst helps to create high yields and high-quality products including gasoline, diesel, kerosene, heating oil, aromatic compounds, and liquefied petroleum gas (LPG). Let us examine how heterogeneous catalysts help in the refining process.

Crude oil is a complex combination of hydrocarbons. In addition, it has small amounts of sulfur and nitrogen-containing organic compounds. As a result, proper processing is necessary for these crude oils. Fractional distillation helps to separate components with distinct boiling point ranges. The naphtha fractions are then processed with hydrodesulfurization (HDS) and hydrodenitrogenation (HDN) catalysts. It assists in removing impurities such as SO2 and NOx precursors. Along with that, reforming, cracking, and hydrocracking are carried out to generate high-grade fuels.
These oil conversion methods rely largely on metals, metal oxides, zeolites, and metal sulfide catalysts. Generally, molybdenum sulfide catalysts help in HDS and HDN. Whereas, catalytic cracking uses zeolites to break down large
hydrocarbon molecules into branched or cyclic hydrocarbons and alkenes. Moreover, catalytic reforming helps noble metals to boost isomerization and cyclization processes without markedly varying the molecular weight of the naphtha fraction.
Environmental Protection
At present, environmental catalysis is often applied in several major commercial sectors. It promotes environmental protection and a higher standard of living. Major uses of these catalysts in environmental protection are; reducing nitrogen oxides (NOx) emissions from stationary sources (power plants, boilers, etc.), processing oil to produce cleaner fuels with low sulfur and nitrogen content, destroying pesticides, dioxins, and furane, controlling the emissions of volatile organic compounds (VOCs), and so on. Furthermore, environmental catalysts have recently found usage in lowering water pollution, oxidizing organic particulates, reducing ozone pollution in urban areas, and improving interior air quality. Finally, they are becoming highly significant in polluted soil decontamination.

Bibliography
- Wang, T., Pan, L., Zhang, X. & Zou, J. Insights into the Pt (111) Surface Aid in Predicting the Selective Hydrogenation Catalyst. Catalysts 10, 1473 (2020).
2. Heveling, J. Heterogeneous Catalytic Chemistry by Example of Industrial Applications. Journel Chem. Educ. 89, 1530−1536 (2012).
3. Ma, Z. & Zaera, F. Heterogeneous Catalysis by Metals. in Encyclopedia of Inorganic Chemistry (2006). doi:10.1002/0470862106.ia084.
4. Glotov, A. & Karakhanov, E. Heterogeneous Catalysts for Petrochemical Synthesis and Oil Refinin. Catalysts 11, 602 (2021).
5. Hagen, J. Heterogeneously Catalyzed Processes in Industry. in IndustrialCatalysis: A Practical Approach 261–298 (2015). doi:10.1002/9783527684625.ch8.
6. Giorgieri, L. . Catalysis For the Protection of the Environment and the Quality of life. (2011).
7. Heterogeneous catalysis – Wikipedia. https://en.wikipedia.org/wiki/Heterogeneous_catalysis.

About the Author
Rosemary Salin is an Associate Chemist at Cademix Institute of Technology, Austria with a Master of Science in Chemistry. Her interests include studying scientific problems and finding different solutions to them through experiments.
Furthermore, she was a recipient of the INSPIRE (Innovation in Science Pursuit for Inspired Research) Scholarship from 2016 to 2021. It is a scholarship program implemented by the Department of Science and Technology (DST), Government of India. It is given to deserving students doing bachelor’s and master’s courses in Natural and Basic Sciences. Moreover, she is a member of the Cademix Career Autopilot Program. She is available for new opportunities. Please feel free to contact her.
E-mails:
rosemary.salin@cademix.org
rosemarysalin99@gmail.com
Linkedin:
Rosemary Salin is an Associate Chemist at Cademix Institute of Technology, Austria with a Master of Science in Chemistry. Her interests include studying scientific problems and finding different solutions to them through experiments.
Furthermore, she was a recipient of the INSPIRE (Innovation in Science Pursuit for Inspired Research) Scholarship from 2016 to 2021. It is a scholarship program implemented by the Department of Science and Technology (DST), Government of India. It is given to deserving students doing bachelor’s and master’s courses in Natural and Basic Sciences. Moreover, she is a member of the Cademix Career Autopilot Program. She is available for new opportunities. Please feel free to contact her.
E-mails:
rosemary.salin@cademix.org
rosemarysalin99@gmail.com
Linkedin:
https://www.linkedin.com/in/rosemary-salin/
Keywords Related To Heterogeneous Catalysis
Catalysis, Homogeneous catalysis, Hydrogenation, Adsorption, Adsorbate, Adsorbent, Desorption, Surface reactions, Physisorption, Chemisorption, Activation energy. Oxo process, Haber-Bosch process, Ostwald’s process, Contact process, and, Ziegler-Natta polymerization.
Crude oil refining, Hydrocarbons, Hydrodesulfurization (HDS), Hydrodenitrogenation (HDN), Cracking, Hydrocracking, Refining, Zeolites, Isomerization, Fractional Distillation.